Peel overwrap at corner and remove solution container. Liquid or powdered medications are provided in a threaded vial that mates with the top. Add vantage bag expiration.
Add Vantage Bag Expiration, The manufacturers instructions for handling and storing ADD-Vantage Mini Bag Plus Add A Vial Add-Ease products and any others should be adhered to. Expiration dates for IV bags out of their overlay wrap. 5 Dextrose Injection USP solution is sterile and nonpyrogenic. A pharmacy compounding service would have to remove the overwrap to add meds and label but this is different from entering the bag with a spike on the admin set or injecting meds into it outside of a laminar airflow workbench.

A debate was started today regarding using IV bags that have been removed from the overwrap. The products discussed in this site may have different product labeling in different countries. The overwrap is a moisture barrier. Drugs can be prepared quickly without needles syringes or alcohol swabs.
License Authorization Form LAF Required.
Read another article:
When I suggested that the bag was still safe to use my colleagues looked at me like I. See USP Controlled Room Temperature Protect from freezing. Each ADD-Vantage vial contains 558 mEq 128 mg of sodium. PRINCIPAL DISPLAY PANEL - 250 mL Bag Overwrap Label - NDC 0409-7101. Also we order Oxytocin 30 unit in 500mL NS bags from PharMEDium and I noticed today that they use Baxter Viaflex 500mL NS bags.
 Source: lazada.com.my
Source: lazada.com.my
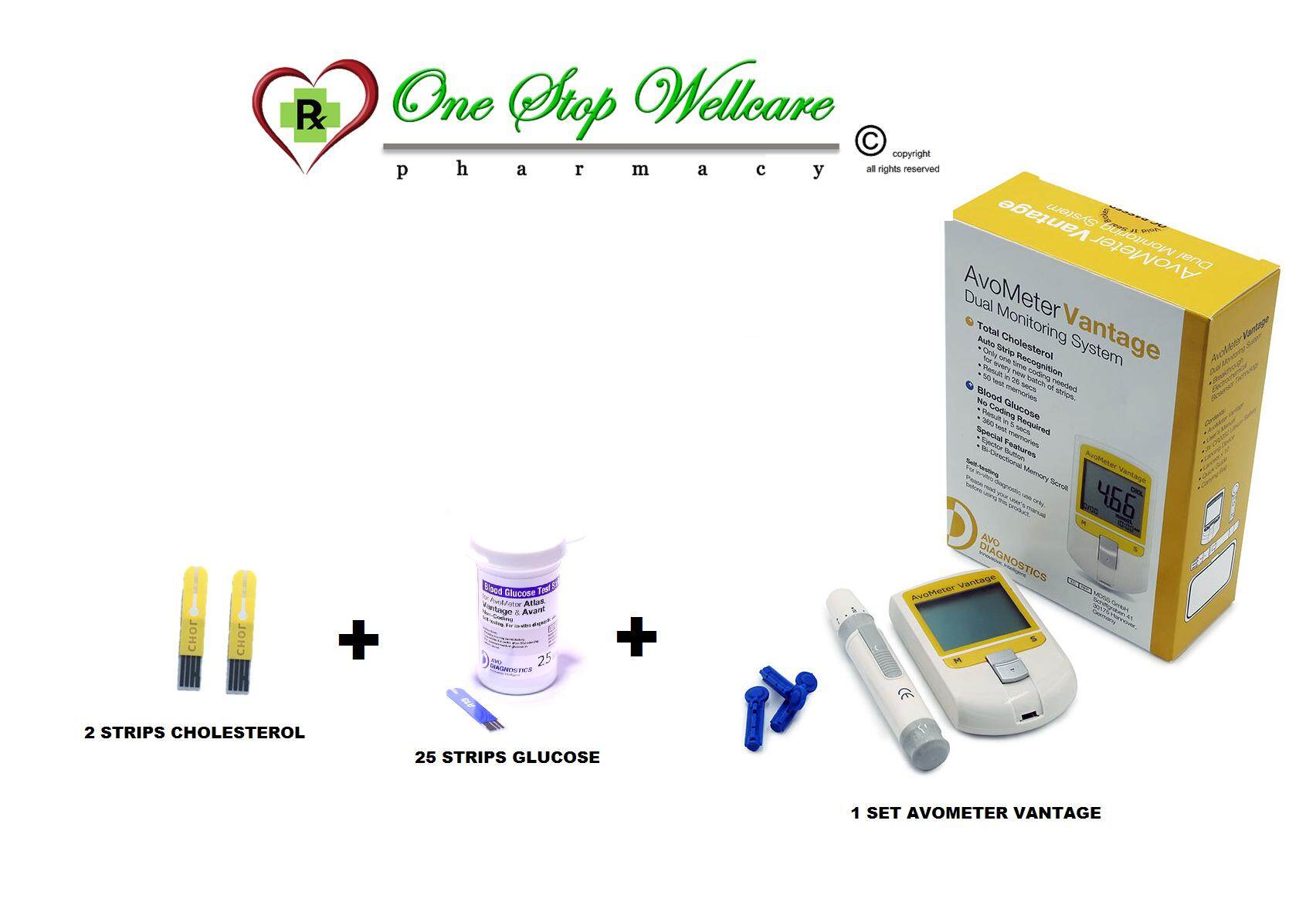
Pfizer for Professionals 1-800-505-4426. 09 sodium chloride injection is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. ADD-Vantage THE SYSTEM 3 1 9-084-CR1-11 2 ADD-Vantage Pull plugstopper to mix drug with diluent ADD-Vantage diluent container is Latex Free and Bar Coded. They have their own overwrap that offers UV protection but its nothing at all like the Baxter overwrap and they assign a much longer expiration date than 30 days. Avometer Vantage Dual Monitoring System 2 Cholesterol Strips 25 Glucose Strips Lazada.
 Source: store.bariatricpal.com
Source: store.bariatricpal.com
Point of Care systems such as Mini-bag Plus Vial Mate ADD-Vantage Varies refer to product package insert for storage and handling Example. My gut also says that would be 1 hour immediate use if made made vial is attached to add-vantage system in a non-sterile area. A variety of factors may be involved in the decision to use premixed RTU and POC IV products. Monitoring Controlled Storage Areas To ensure that product potency is retained through the manufacturers labeled expiration date compounding personnel should monitor and document the drug storage areas within the compounding facility. Bariatric Advantage Floravantage Balance Probiotic 15 Billion Cfu Capsules 60 Count.

Proprietary BagVial Systems Mini-Bag Plus ADD-Vantage Vial-Mate Adaptors Add-a-Vial. PRINCIPAL DISPLAY PANEL - 250 mL Bag Overwrap Label - NDC 0409-7101. Products are FDA-Approved - follow manufacturers instructions for handling and storing. License Authorization Form LAF Required. Diltiazem Add Vantage Solutions Emergency Medical Products.
 Source: militaryworldsrl.com
Source: militaryworldsrl.com
These ADD-Vantage vials by Hospira contain sodium chloride that is designed solely for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection. The products discussed in this site may have different product labeling in different countries. I work in an OR we use a lot of IVs in a day. They have their own overwrap that offers UV protection but its nothing at all like the Baxter overwrap and they assign a much longer expiration date than 30 days. Hawke Vantage 4 16x50 Ao Mil Dot Illuminato 392921targ.
 Source: faq.vantagecrypto.com
Source: faq.vantagecrypto.com
INSTRUCTIONS FOR USE WITH ADD-VANTAGE VIAL. My gut also says that would be 1 hour immediate use if made made vial is attached to add-vantage system in a non-sterile area. Liquid or powdered medications are provided in a threaded vial that mates with the top. License Authorization Form LAF Required. Vantagecrypto Faq S.

The manufacturers instructions for handling and storing ADD-Vantage Mini Bag Plus Add A Vial Add-Ease products and any others should be adhered to. I called them today and should receive an email. Sodium Chloride 09 ADD-Vantage diluent bags. When I suggested that the bag was still safe to use my colleagues looked at me like I. 2.
 Source: dailymed.nlm.nih.gov
Source: dailymed.nlm.nih.gov
Use unit within 30 days of opening overwrap as long as the use date does not exceed the printed expiration date. It is a parenteral solution containing 50 mgmL of dextrose hydrous in water for injection and is intended for intravenous administration after admixing with an ADD-Vantage vial or single-dose powdered drug vials with 20 mm closure using the ADD-Vantage ADDAPTOR TM WARNING. Non-sterile Multi dose containers Manufacturers expiration date unless otherwise specified by manufacturer Non-sterile compounded products USP 795 June 2013. A pharmacy compounding service would have to remove the overwrap to add meds and label but this is different from entering the bag with a spike on the admin set or injecting meds into it outside of a laminar airflow workbench. Sodium Chloride.
 Source: dailymed.nlm.nih.gov
Source: dailymed.nlm.nih.gov
Liquid or powdered medications are provided in a threaded vial that mates with the top. These instructions for use should be made available to the individuals who perform the reconstitution steps. Zosyn piperacillin and tazobactam for injection is also supplied in the ADD-Vantage Vial as follows. Quickly create one account for all PfizerPro resources check sample availability and eligibility and receive communications from Pfizer about our products. Sodium Chloride.
 Source: faq.vantagecrypto.com
Source: faq.vantagecrypto.com
License Authorization Form LAF Required. ADD-Vantage THE SYSTEM 3 1 9-084-CR1-11 2 ADD-Vantage Pull plugstopper to mix drug with diluent ADD-Vantage diluent container is Latex Free and Bar Coded. INSTRUCTIONS FOR USE WITH ADD-VANTAGE VIAL. Screw the vial into the vial port until it will go no further. Vantagecrypto Faq S.
 Source: nutritionwarehouse.com.au
Source: nutritionwarehouse.com.au
A pharmacy compounding service would have to remove the overwrap to add meds and label but this is different from entering the bag with a spike on the admin set or injecting meds into it outside of a laminar airflow workbench. I called them today and should receive an email. Quickly create one account for all PfizerPro resources check sample availability and eligibility and receive communications from Pfizer about our products. Add-Vantage Replacement Preparation Sodium Chloride 09 IV Solution Flexible Bag 250 mL Hospira 00409710102. Wrist Support With Wrist Loop By Vantage Strength Accessories Big Brands Warehouse Prices.
 Source: dailymed.nlm.nih.gov
Source: dailymed.nlm.nih.gov
A debate was started today regarding using IV bags that have been removed from the overwrap. Examples include ADD-Vantage Mini-Bag Plus Duplex and Vial-Mate. ADD-Vantage keeps drug and diluent separate until the system is activated just prior to intravenous administration. 09 sodium chloride injection is a sterile nonpyrogenic isotonic solution of sodium chloride and water for injection. Sodium Chloride.
 Source: fleetsafety.com
Source: fleetsafety.com
Products are FDA-Approved - follow manufacturers instructions for handling and storing. Quickly create one account for all PfizerPro resources check sample availability and eligibility and receive communications from Pfizer about our products. A pharmacy compounding service would have to remove the overwrap to add meds and label but this is different from entering the bag with a spike on the admin set or injecting meds into it outside of a laminar airflow workbench. Drugs can be prepared quickly without needles syringes or alcohol swabs. Streamlight 88901 Vantage 180 X Includes Two Cr123a Lithium Batteries Helmet Bracket Box Orange Fleet Safety.
 Source: dailymed.nlm.nih.gov
Source: dailymed.nlm.nih.gov
A pharmacy compounding service would have to remove the overwrap to add meds and label but this is different from entering the bag with a spike on the admin set or injecting meds into it outside of a laminar airflow workbench. 250 mL bags NDC 0409-7101-66 Case of 50 NDC 0409-7101-68 50 mL bags NDC 0409-7101-67 Case of 50 09 NDC 0409-7101-69 100 mL bags NDC 0409-7101-02 Case of 24 09 NDC 0409-7101-04 250 mL bags Store at 20 to 25C 68 to 77F. I called them today and should receive an email. Always follow your local protocols regarding product use. Sodium Chloride.
 Source: faq.vantagecrypto.com
Source: faq.vantagecrypto.com
INSTRUCTIONS FOR USE WITH ADD-VANTAGE VIAL. OneADD-Vantage Unit TO OPENPEEL AT CORNER. These ADD-Vantage vials by Hospira contain sodium chloride that is designed solely for parenteral use only after addition of drugs that require dilution or must be dissolved in an aqueous vehicle prior to injection. Quickly create one account for all PfizerPro resources check sample availability and eligibility and receive communications from Pfizer about our products. Vantagecrypto Faq S.

Due to manufacturer requirements this product is no longer available to be ordered in the EACH quantity. See USP Controlled Room Temperature Protect from freezing. Due to manufacturer requirements this product is no longer available to be ordered in the EACH quantity. Expiration dates for IV bags out of their overlay wrap. 2.







